Vitamin K Im Injection Site

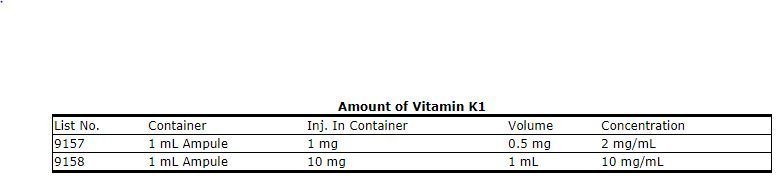
Each milliliter contains phytonadione 2 or 10 mg polyoxyethylated fatty acid derivative 70 mg dextrose hydrous 37 5 mg in water for injection.
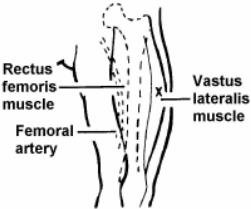
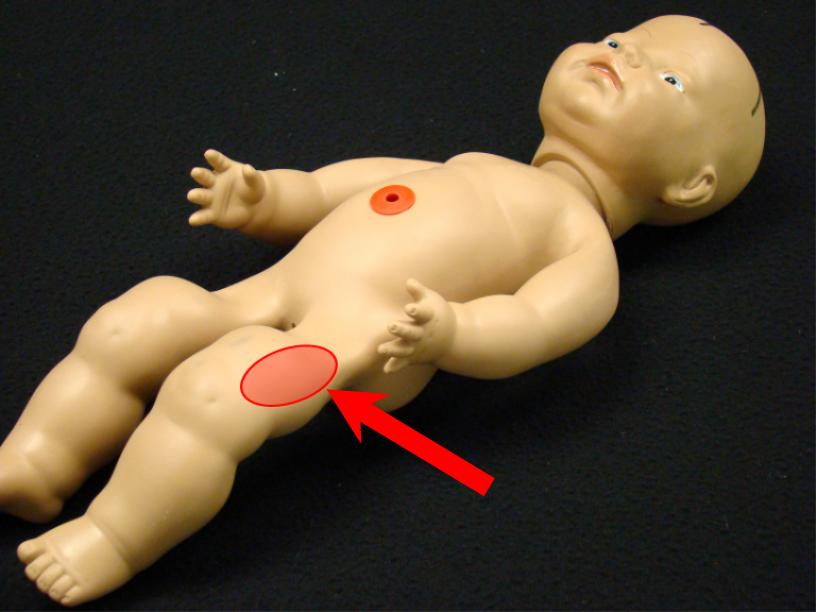
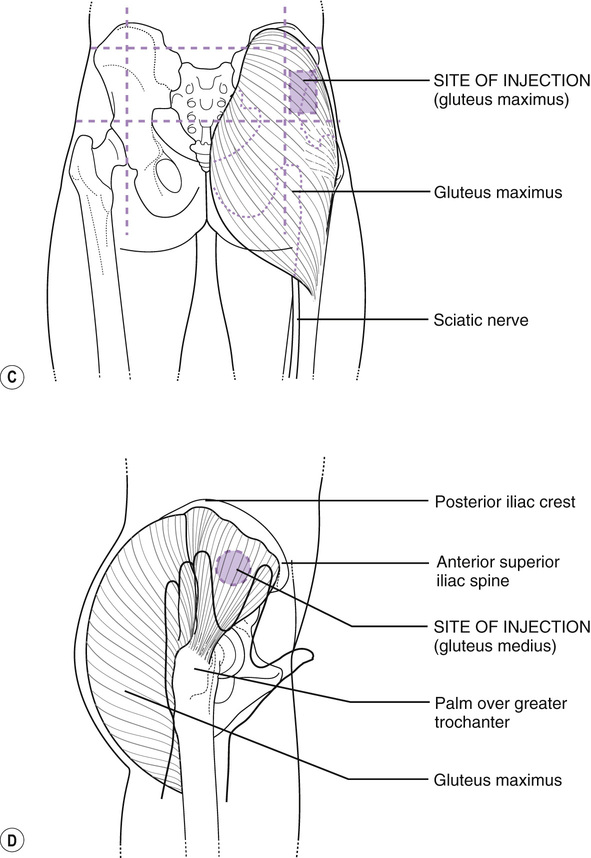
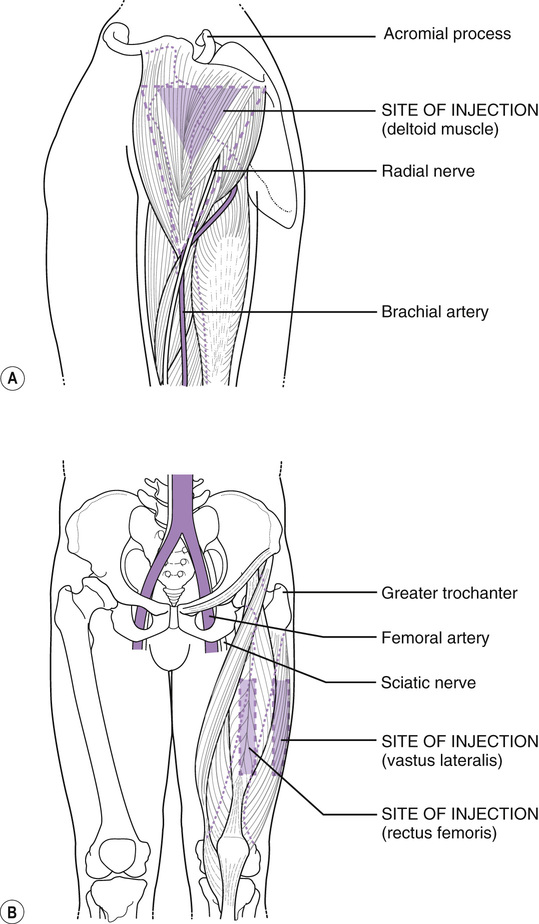
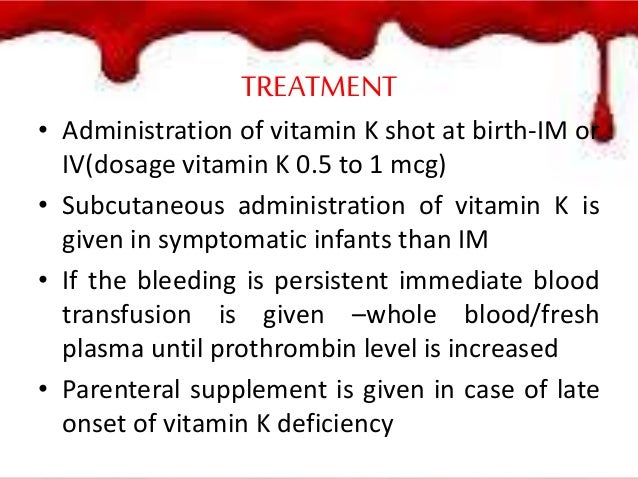
Vitamin k im injection site. Adults and teenagers the usual dose is 5 to 15 mg injected into a muscle or under the skin one or two times a day. This medication is given by injection under the skin or into a muscle or vein as directed by your doctor. The anterolateral thigh is the preferred site for im injection in infants under 12 months of age. The frequency of refusal of im vitamin k at individual sites ranged from none 1 site in ny and 1 site in al to 2 3 1 site in or.
Nested patient control study to assess associations between refusal of im vitamin k administration and various factors we enrolled 195 patients and 985 controls. Inr reduction observed within 24 48 hr monitor inr and give additional vitamin k if needed. 10 mg ml 1 mg 0 5 ml how should i keep vitamin k injection stored. 2012 accp guidelines recommend vitamin k1 po dose not specified.
Vitamin k1 injection phytonadione injectable emulsion usp is a yellow sterile nonpyrogenic aqueous dispersion available for injection by the intravenous intramuscular and subcutaneous routes. Consider 2 5 5 mg po once. Tablets should be stored at room temperature between 15 c and 30 c 59 f and 86 f. How to use vitamin k1 ampul this medication is given by injection under the skin or into a muscle or vein as directed by your doctor.
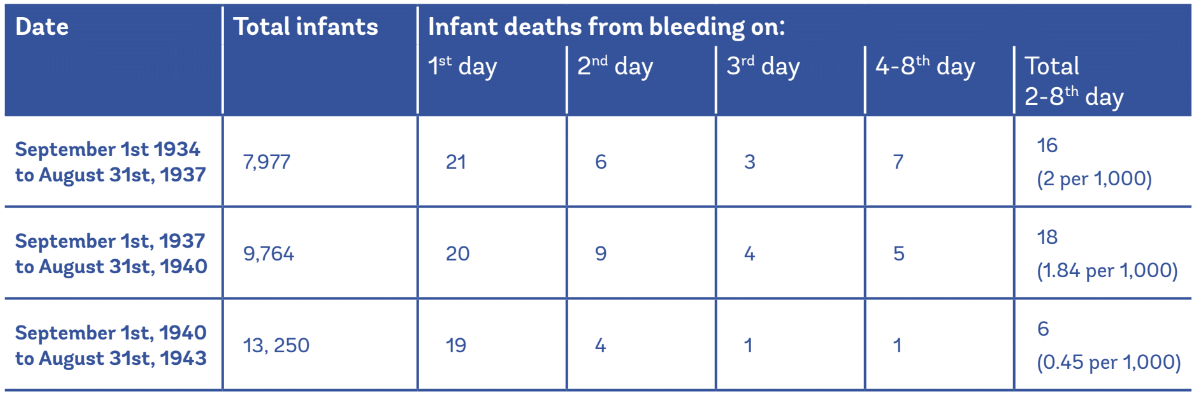
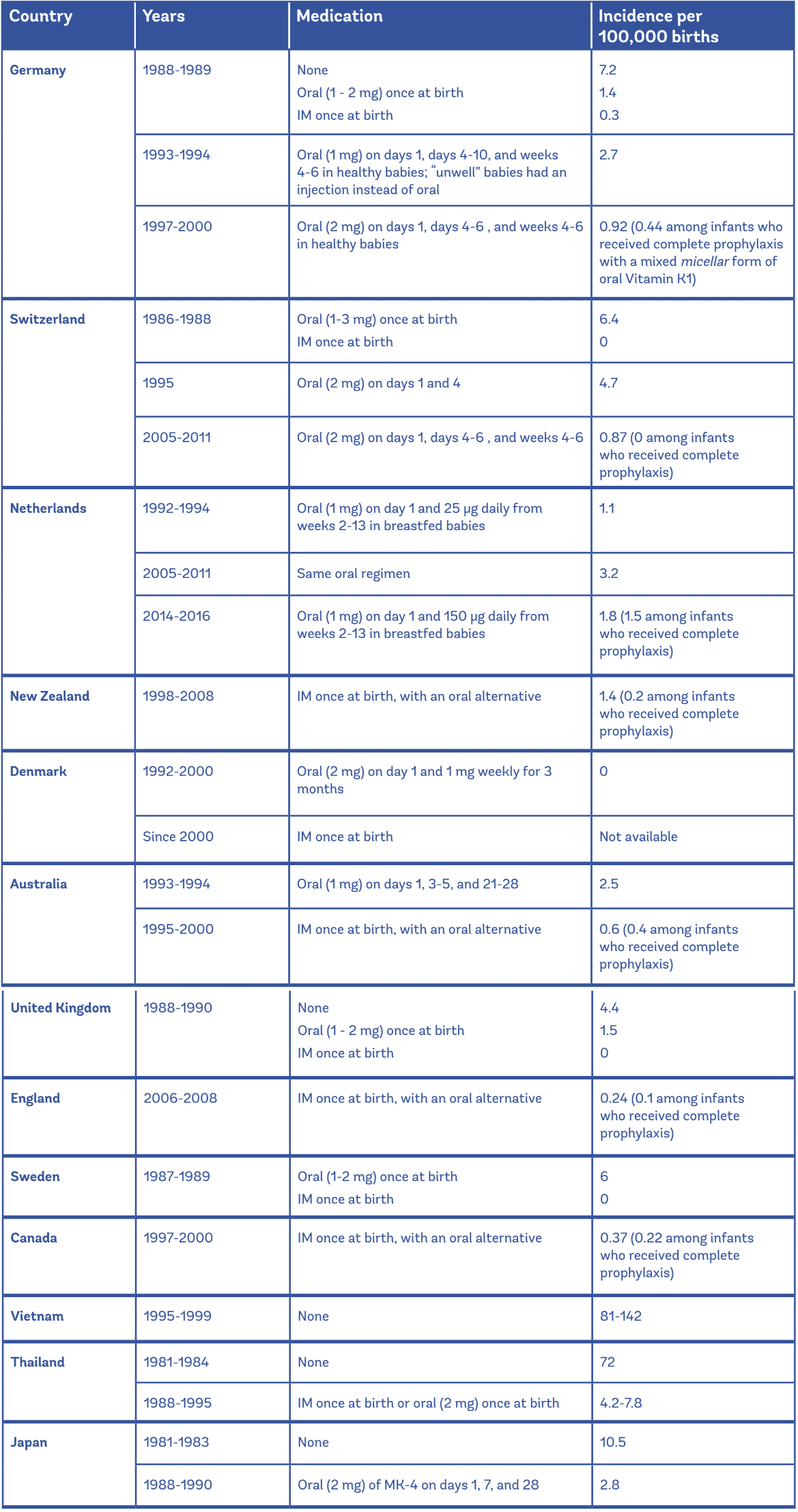
Oral administration of a single dose of vitamin k protects against classical and early vkdb but is less effective than intramuscular im prophylaxis for the prevention of late vkdb. Minor bleeding any elevated inr. We reviewed the literature on the frequency of reported reasons for and factors associated with refusal of. Benzyl alcohol 9 mg added as preservative.
If this medication is given into a vein it should be injected very slowly no more than 1. Inr 10 no bleeding. In 2019 the american academy of pediatrics made public education about intramuscular vitamin k administration at birth a public health priority partly in response to reports of refusal of intramuscular vitamin k by parents of newborns that led to vitamin k deficiency bleeding vkdb. 2008 accp guidelines suggest 2 5 5 mg po once.
May repeat if needed after 24 hr. Injectable vitamin k should be stored in original container at controlled room temperature between 20 c and 25 c 68 f and 77 f and should be protected from light. Although an increased risk of solid tumour associated vitamin k administration can be definitively excluded a low potential risk of lymphoblastic leukaemia in childhood can not be ruled out.